The partner of AffaMed Therapeutics (“AffaMed”), Ocular Therapeutix, Inc. (NASDAQ:OCUL), a biopharmaceutical company focused on the formulation, development, and commercialization of innovative therapies for diseases and conditions of the eye, recently announced the U.S. Food and Drug Administration (FDA) has approved its Supplemental New Drug Application (sNDA) to broaden the DEXTENZA ® label to add an additional indication for the treatment of ocular itching associated with allergic conjunctivitis.
According to the statistics, an estimated 10 million1,2,3 people in the U.S. annually seek medical attention for the inflammatory response associated with allergic conjunctivitis caused by both seasonal and perennial allergens. In China, the prevalence of allergic conjunctivitis is estimated to be as high as 10%, which implies one in ten Chinese who may suffer from allergic conjunctivitis. The figures in the US and China represent a discrete market opportunity for DEXTENZA beyond its current use in the surgical setting.
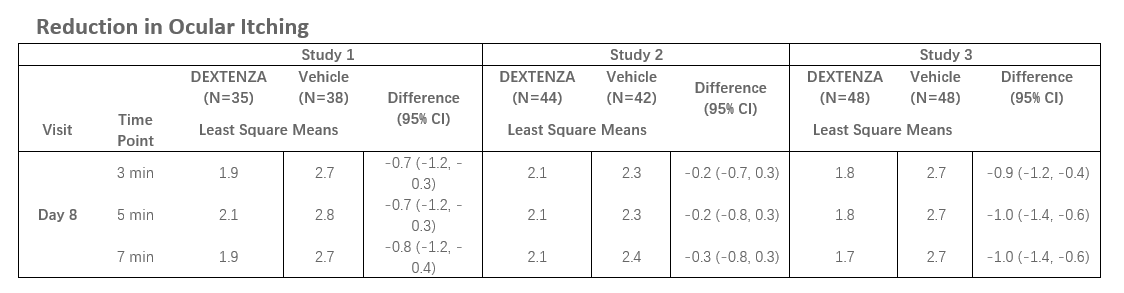
The efficacy of DEXTENZA for the treatment of ocular itching associated with allergic conjunctivitis was based on three randomized, multicenter, double-masked, parallel group, vehicle-controlled studies in subjects with a positive history of ocular allergies and positive skin test reaction to perennial and seasonal allergens (n=255). In all three trials, DEXTENZA demonstrated lower mean ocular itching scores compared with the vehicle group at all time points throughout the study duration of up to 30 days. In two of the three studies, a higher proportion of patients had statistically significant reductions in ocular itching on Day 8, at 3 minutes, 5 minutes and 7 minutes post-challenge in the DEXTENZA group compared to the vehicle group. Data for the primary endpoint, ocular itching at Day 8, is shown below for all three studies (scale 0-4):
With the approval, DEXTENZA is the first, FDA-approved, physician-administered intracanalicular insert capable of delivering a preservative-free drug for the treatment of ocular itching associated with allergic conjunctivitis with a single administration for up to 30 days. DEXTENZA originally received FDA approval in November 2018 for the treatment of ocular pain following ophthalmic surgery, followed by an expansion of the label to also include the treatment of ocular inflammation following ophthalmic surgery in June 2019.
AffaMed reached the license agreement with Ocular in October,2020, and has the exclusive rights to develop and commercialize DEXTENZA for the treatment of post-surgical inflammation and pain following ophthalmic surgery and ocular itching in patients with allergic conjunctivitis in Greater China, which includes mainland China, Hong Kong, Macau, and Taiwan; South Korea; and the ASEAN Countries (Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand and Vietnam). Currently, AffaMed is working closely with NMPA to explore the pathway for DEXTENZA to accelerate the registration process in China.
1. Leonardi A, Castegnaro A, Valerio ALG, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15(5):482-488.
2. Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2011;11(5):471-476
3. Ora website, An Update on Ocular Allergy Trends, 2019 Ora, Inc., www.oraclinical.com
About DEXTENZA
DEXTENZA is FDA approved for the treatment of ocular inflammation and pain following ophthalmic surgery and ocular itching associated with allergic conjunctivitis. DEXTENZA is a corticosteroid intracanalicular insert placed in the punctum, a natural opening in the inner portion of the lower eyelid, and into the canaliculus and is designed to deliver dexamethasone to the ocular surface for up to 30 days without preservatives. DEXTENZA resorbs and exits the nasolacrimal system without the need for removal.

